Pharmaceutical company, Pfizer, have announced that early results from its coronavirus vaccine suggest the shots may be 90% effective at preventing COVID-19.
Pfizer and German partner, BioNTech SE, are the first drug-makers to show successful data from a large-scale clinical trial of a coronavirus vaccine, putting them on track to apply for emergency-use approval from the US Food and Drug Administration later this month.
If authorised, the number of vaccine doses will initially be limited. Many questions also remain, including how long the vaccine will provide protection.
READ MORE: Mitsotakis reassures citizens of Greece’s plan to have Covid-19 vaccine by end of December.

However, the news provides hope that other vaccines in development against the coronavirus may also prove effective.
“Today is a great day for science and humanity,” Albert Bourlas, Pfizer’s chairman and chief executive, said in a statement.
“We are reaching this critical milestone in our vaccine development program at a time when the world needs it most with infection rates setting new records, hospitals nearing over-capacity and economies struggling to reopen.”
READ MORE: COVID-19 vaccine: Greek and Australian PM’s give mixed messages.

Speaking later on CNBC, Bourlas added that the vaccine was “a light at the end of the tunnel.”
“When you realise your vaccine has 90% effectiveness, that’s overwhelming,” Mr Bourlas said.
“I am very happy but at the same time, sometimes I have tears in my eyes when I realise that this is the end of nine months, day-and-night work of so many people and how many people, billions, invested hopes on this. I never thought it would be 90%.”
READ MORE: Greek PM: “Let philotimo be our national vaccine” against coronavirus.
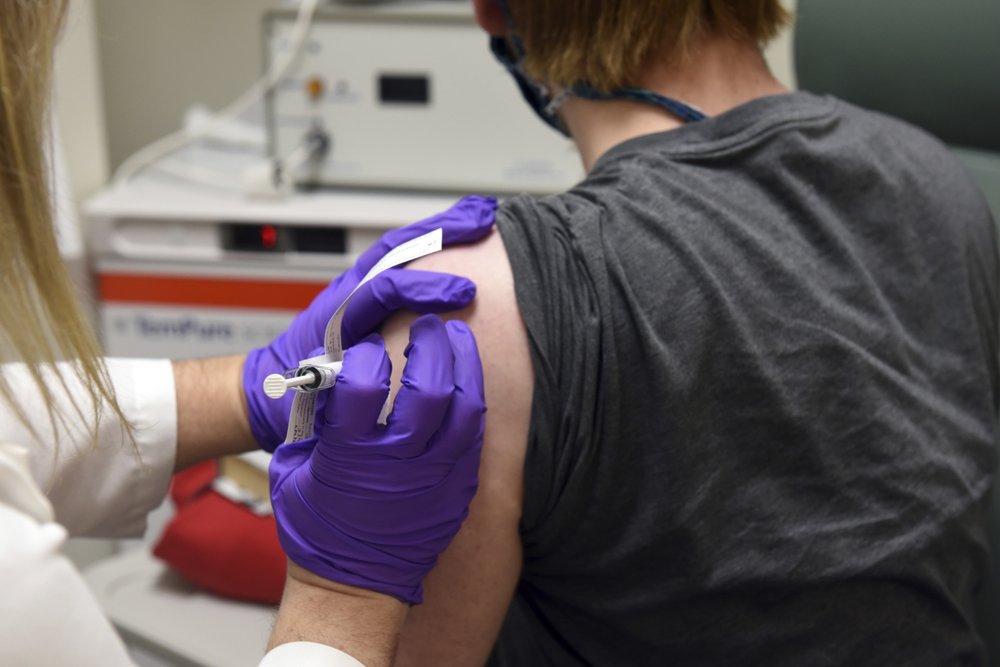
Pfizer said the interim analysis was conducted after 94 participants in the trial developed COVID-19, examining how many of them received the vaccine versus a placebo.
The company did not break down exactly how many of those who fell ill received the vaccine. Still, over 90% effectiveness implies that no more than 8 of the 94 people who caught COVID-19 had been given the vaccine, which was administered in two shots about three weeks apart.
The efficacy rate is well above the 50% effectiveness required by the US Food and Drug Administration for a coronavirus vaccine.
READ MORE: Australian religious leaders raise “ethical concerns” about potential COVID-19 vaccine.

Pfizer and BioNTech have a $1.95 billion contract with the US government to deliver 100 million vaccine doses beginning this year. They have also reached supply agreements with the European Union, the UK, Canada and Japan.
To save time, the companies began manufacturing the vaccine before they knew whether it would be effective. They now expect to produce up to 50 million doses or enough vaccine to protect 25 million people this year.
Pfizer said it expects to produce up to 1.3 billion doses of the vaccine in 2021.
