In December 2013, Foutoula Maitianos was diagnosed with Multiple Sclerosis (MS) – a condition of the central nervous system that affects mobility and can cause fatigue and memory loss.
Almost seven years later in 2020, the South Australian mum-of-two saw her condition deteriorate significantly to the point where she relied on a walking frame or wheelchair.
“My walking deteriorated, I started getting headaches, fatigue, it was becoming a struggle,” Ms Maitianos told ABC News.
Ms Maitianos knew the goal for people living with MS was to stop symptoms from getting worse, but none of her treatments were doing that.
“I was worried because I was on a few different treatments and I was still deteriorating,” she says, explaining she spent 18 months on intravenous drug Ocrevus (Ocrelizumab).
Her hopes turned to an experimental treatment called autologous haematopoietic stem cell transplant (AHSCT).

What is AHSCT?
AHSCT is a procedure that involves harvesting stem cells from patients, giving them doses of chemotherapy, then replacing the stem cells in the patient’s body. The aim is to ‘reset’ the immune system to stop it attacking the body.
According to MS Research Australia, the nation’s leading non-profit organisation for MS research and advocacy, AHSCT carries a higher risk than most currently approved therapies.
“We know that AHSCT can have some significant effects on the body such as a high risk of severe infections, effects on fertility, and an increased risk of cancers,” the organisation said in a statement to ABC News.
It said while there had been no deaths among about 50 people who have received AHSCT in Australia in the past eight years, the average mortality rate from international studies is 1.3 per cent.
Despite this, Ms Maitianos felt like AHSCT was still her best option.

The only problem was that the treatment was only available in Australia through three observational clinical trials at Sydney’s St Vincent’s Hospital and in Melbourne at Austin Health and The Alfred Hospital.
Ms Maitianos’ doctors warned she may not be eligible but as the treatment was available in several countries overseas, she decided to head to Mexico despite the raging COVID-19 pandemic.
“It was scary, very scary, but I knew I had to do it, I didn’t care what was going on,” she told ABC News.
On a mission to Mexico:
Ms Maitianos and her husband, Peter, left for Mexico in October 2020, making provisions for their children while they were away for six weeks.
They were supported by friends, family and the wider community in fundraising to cover the costs of the trip and medical treatment. They also had to get an exemption to be able to leave Australia in the first place.
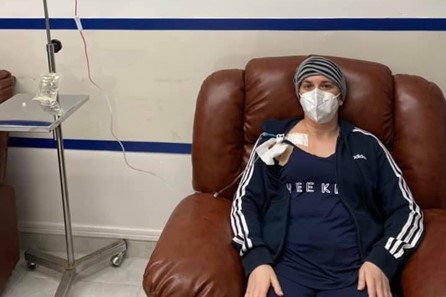
“It was crazy because my family and friends were saying ‘Are you sure you want to go?’ because we knew there were a lot more cases [of COVID-19] than in Australia,” Ms Maitianos says.
But still, she has no regrets as once she reached the Mexican treatment facility, she was able to not only receive AHSCT, but also connect with fellow MS patients from across the world.
“There were 14 to 15 of us and because of COVID we all had to stay in, we couldn’t leave the facility, we got along so well… It was great to have the support,” Ms Maitianos told ABC News.
After returning to Australia, Ms Maitianos quarantined in Sydney for two weeks before isolating herself at home for several months due to her low immune system.
And while it is too soon to know whether the treatment has worked, Ms Maitianos feels it was the right choice for her.
“My hope is that it just stops in its tracks where it’s at, that’s the whole point of this treatment,” she says. “For now I’m very hopeful. It is a rollercoaster the first few years after treatment.”
Source: ABC News.

